Photochemical and -dynamic therapy implant
PCDTI
The fully implantable PCDTI device consists of an intracerebral radiation-emitting implant, a transcutaneously rechargable power supply, a control unit and connective wiring. At the end of glioblastoma resection, i.e., within the same surgical session, the intracerebral device is size-adapted to fit the dimensions of the resection cavity, allowing the postoperative long-term repetitive delivery of radiation with different wavelengths and different energies (thus different tissue penetration depths and different biological effects) to the tumor infiltration zone adjacent to the resection cavity wall. The biological radiation effects are partly mediated (or augmented) by a photosensitizing molecule (intracellularly created from an orally bio-available pro-drug) that specifically accumulates in tumor cells.
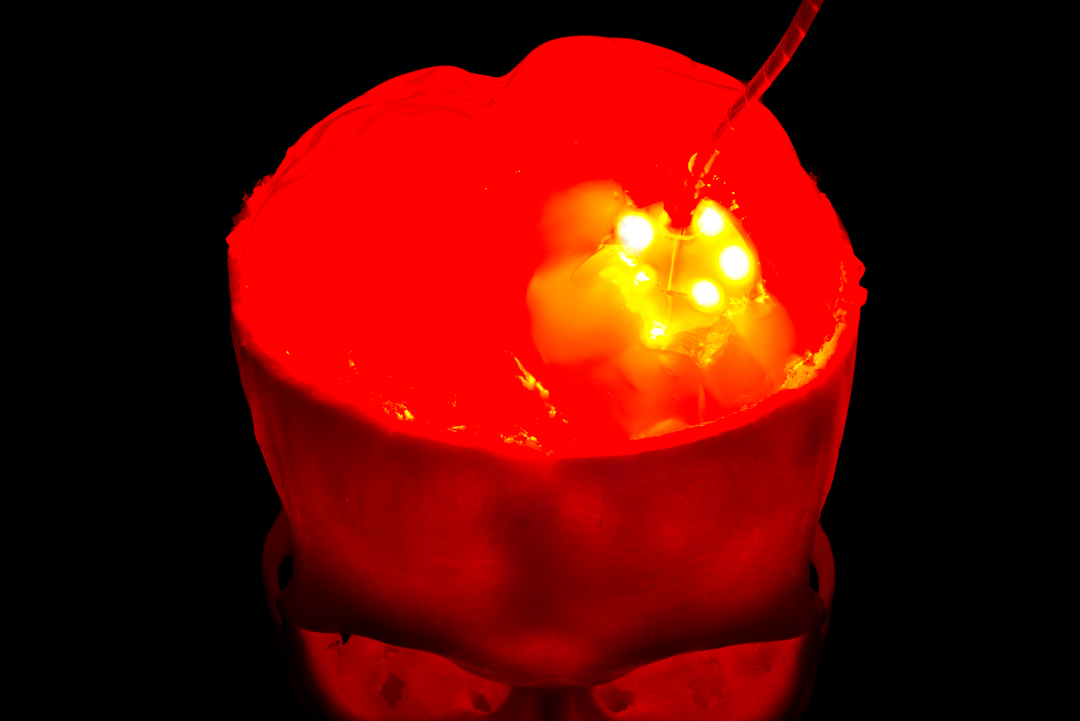
The wavelengths of the radiation emitted by the implant are more than 100-fold above that of X-rays, underlining that the methodology used has little in common with traditional radiation therapy.
The fact that following gross total resection the vast majority of glioblastoma recurrences occur within a 2-cm rim adjacent to the resection cavity wall has given rise to the concept of so-called supramarginal tumor resection extending 1-2 cm beyond the margins of contrast-enhancing glioblastoma. However, the practical applicability of supramarginal tumor resection may be largely limited to non-eloquent glioblastoma locations such as the temporo-polar or non-dominant frontal regions. In eloquent locations of the tumor infiltration zone, on the other hand, long-term repetitive treatment by the PCDTI may be particularly advantageous. Irrespective of location, the goal of treatment with the PCDTI is a significant delay of glioblastoma recurrence.
In our preclinical tolerability study, both the surgical device implantation as well as repetitive intracranial implant activation (using manifold the doses determined to be cytotoxic to glioblastoma cells in vitro) were well-tolerated. We are now planning a preclinical effectivity study in an orthotopic xenotransplanted porcine model of human glioblastoma.